Long term efficacy, safety, and tolerability of tildrakizumab in epileptic patient with psoriasis and eczema
Accepted: 16 September 2022
HTML: 32
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
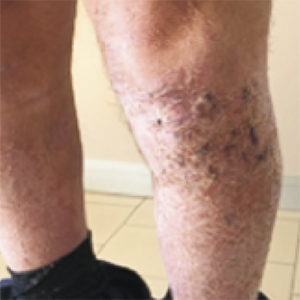
Psoriasis is a chronic inflammatory disease which mostly affects skin. Tildrakizumab is a specific anti-interleukin-23p19 monoclonal antibody approved for the treatment of plaque psoriasis in adults. Herein, we report about a patient who came to our attention for a moderate-to-severe plaque psoriasis, involving primarily upper limbs, elbow, abdomen and knees (PASI 18 – DLQI 22). His medical history was relevant for epilepsy controlled pharmacologically. In addition, an eczematous and edematous appearance of the tibial area was detected; the histologic findings did not contradict the diagnostic hypothesis of subacute spongiotic dermatitis. The patient was treated with Tildrakizumab. After 12 weeks the clinical lesions improved significantly, and the eczematous component disappeared in the tibial area bilaterally. The clinical improvement was maintained even after one year of therapy. Tildrakizumab showed excellent results in the control of psoriasis, with an excellent safety profile. The promising results of clinical trials have been confirmed in a real-life setting. There are no reports about its safety in epileptic patients. In our case, neurological adverse events did not verify and tildrakizumab managed to control both the psoriatic and eczematous components
AlQassimi S, AlBrashdi S, Galadari H, Hashim MJ. Global burden of psoriasis - comparison of regional and global epidemiology, 1990 to 2017. Int J Dermatol. 2020;59(5):566-571. DOI: https://doi.org/10.1111/ijd.14864
Rendon A, Schäkel K. Psoriasis Pathogenesis and Treatment. Int J Mol Sci. 2019;20(6):1475. Published 2019 Mar 23. DOI: https://doi.org/10.3390/ijms20061475
Sivamani RK, Goodarzi H, Garcia MS, et al. Biologic therapies in the treatment of psoriasis: a comprehensive evidence-based basic science and clinical review and a practical guide to tuberculosis monitoring. Clin Rev Allergy Immunol. 2013;44(2):121-140. DOI: https://doi.org/10.1007/s12016-012-8301-7
Reich K, Warren RB, Iversen L, et al. Long-term efficacy and safety of tildrakizumab for moderate-to-severe psoriasis: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2) through 148 weeks. Br J Dermatol. 2020;182(3):605-617. DOI: https://doi.org/10.1111/bjd.18232
Tildrakizumab, Summary of Product Characteristics
Kopp T, Riedl E, Bangert C, et al. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature. 2015;521(7551):222-226. DOI: https://doi.org/10.1038/nature14175
Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials [published correction appears in Lancet. 2017 Jul 15;390(10091):230]. Lancet. 2017;390(10091):276-288. DOI: https://doi.org/10.1016/S0140-6736(17)31279-5
Drerup KA, Seemann C, Gerdes S, Mrowietz U. Effective and Safe Treatment of Psoriatic Disease with the Anti-IL-23p19 Biologic Tildrakizumab: Results of a Real-World Prospective Cohort Study in Nonselected Patients [published online ahead of print, 2021 Nov 12]. Dermatology. 2021;1-5. DOI: https://doi.org/10.1159/000519924
Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129(6):1339-1350. DOI: https://doi.org/10.1038/jid.2009.59
Mao LY, Ding J, Peng WF, et al. Interictal interleukin-17A levels are elevated and correlate with seizure severity of epilepsy patients. Epilepsia. 2013;54(9):e142-e145. DOI: https://doi.org/10.1111/epi.12337
Barry K, Zancanaro P, Casseres R, Abdat R, Dumont N, Rosmarin D. Concomitant atopic dermatitis and psoriasis - a retrospective review. J Dermatolog Treat. 2021;32(7):716-720. DOI: https://doi.org/10.1080/09546634.2019.1702147
Husein-ElAhmed H, Steinhoff M. Effectiveness of ustekinumab in patients with atopic dermatitis: analysis of real-world evidence [published online ahead of print, 2021 Aug 25]. J Dermatolog Treat. 2021;1-6. DOI: https://doi.org/10.1080/09546634.2021.1914315
Copyright (c) 2022 the Author(s)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.





 https://doi.org/10.4081/dr.2022.9447
https://doi.org/10.4081/dr.2022.9447



