Ustekinumab in the treatment of acute disseminated pyoderma gangrenosum in a patient with Crohn’s disease
Accepted: 3 February 2023
HTML: 32
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
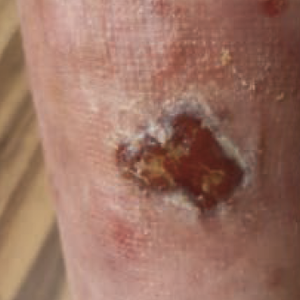
Pyoderma gangrenosum (PG) is an auto-inflammatory dermatosis characterized by lesions that often cause ulcers. We present a case of successful ustekinumab treatment for acute general PG in a 31-year-old woman with coexisting Crohn’s disease (CD). For a month, the patient suffered from skin ulcers, two of them deep and necrotic; a histopathological examination revealed PG. Treatment included: methylprednisolone, azathioprine, betamethasone, gentamicin and zincic ointments, antiseptic compresses, and adalimumab therapy. Due to resistance to the implemented treatment, the patient was enrolled in a clinical trial that included the administration of an anti-cytokines drug, ustekinumab. Subsequently, a significant reduction was observed in the severity of symptoms of PG with no relapse. The use of ustekinumab in patients with PG who have an inadequate response to current treatment or cannot receive first-line treatment can be considered. This applies especially to patients with accompanying autoimmune diseases such as CD.
Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol [Internet]. 2012 [cited 2022 Mar 9];13(3):191–211. Available from: https://pubmed.ncbi.nlm.nih.gov/22356259/ DOI: https://doi.org/10.2165/11595240-000000000-00000
Maverakis E, Marzano A v., Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers [Internet]. 2020 Dec 1 [cited 2022 Mar 9];6(1). Available from: https://pubmed.ncbi.nlm.nih.gov/33033263/ DOI: https://doi.org/10.1038/s41572-020-0213-x
Plumptre I, Knabel D, Tomecki K. Pyoderma Gangrenosum: a review for the gastroenterologist. Inflamm Bowel Dis [Internet]. 2018 Dec 1 [cited 2022 Mar 9];24(12):2510–7. Available from: https://pubmed.ncbi.nlm.nih.gov/29788368/ DOI: https://doi.org/10.1093/ibd/izy174
George C, Deroide F, Rustin M. Pyoderma gangrenosum - a guide to diagnosis and management. Clin Med (Lond) [Internet]. 2019 May 1 [cited 2022 Mar 9];19(3):224–8. Available from: https://pubmed.ncbi.nlm.nih.gov/31092515/ DOI: https://doi.org/10.7861/clinmedicine.19-3-224
Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol [Internet]. 2018 Apr 1 [cited 2022 Mar 9];154(4):461–6. Available from: https://pubmed.ncbi.nlm.nih.gov/29450466/ DOI: https://doi.org/10.1001/jamadermatol.2017.5980
Alavi A, French LE, Davis MD, et al. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol [Internet]. 2017 Jun 1 [cited 2022 Mar 9];18(3):355–72. Available from: https://pubmed.ncbi.nlm.nih.gov/28224502/ DOI: https://doi.org/10.1007/s40257-017-0251-7
Miller J, Yentzer BA, Clark A, et al. Pyoderma gangrenosum: a review and update on new therapies. J Am Acad Dermatol [Internet]. 2010 Apr [cited 2022 Mar 9];62(4):646–54. Available from: https://pubmed.ncbi.nlm.nih.gov/20227580/ DOI: https://doi.org/10.1016/j.jaad.2009.05.030
Herberger K, Dissemond J, Brüggestrat S, et al. Biologics and immunoglobulins in the treatment of pyoderma gangrenosum - analysis of 52 patients. J Dtsch Dermatol Ges [Internet]. 2019 Jan 1 [cited 2022 Aug 3];17(1):32–41. Available from: https://pubmed.ncbi.nlm.nih.gov/30592563/ DOI: https://doi.org/10.1111/ddg.13741
Benson JM, Peritt D, Scallon BJ, et al. Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs [Internet]. 2011 Nov [cited 2022 Mar 9];3(6). Available from: https://pubmed.ncbi.nlm.nih.gov/22123062/ DOI: https://doi.org/10.4161/mabs.3.6.17815
Agarwal A, Andrews JM. Systematic review: IBD-associated pyoderma gangrenosum in the biologic era, the response to therapy. Aliment Pharmacol Ther [Internet]. 2013 Sep [cited 2022 Mar 9];38(6):563–72. Available from: https://pubmed.ncbi.nlm.nih.gov/23914999/ DOI: https://doi.org/10.1111/apt.12431
Langan SM, Groves RW, Card TR, Gulliford MC. Incidence, mortality, and disease associations of pyoderma gangrenosum in the United Kingdom: a retrospective cohort study. J Invest Dermatol [Internet]. 2012 [cited 2022 Mar 9];132(9):2166–70. Available from: https://pubmed.ncbi.nlm.nih.gov/22534879/ DOI: https://doi.org/10.1038/jid.2012.130
Marzano AV, Trevisan V, Lazzari R, Crosti C. Pyoderma gangrenosum: study of 21 patients and proposal of a “clinicotherapeutic” classification. J Dermatolog Treat [Internet]. 2011 Oct [cited 2022 Mar 9];22(5):254–60. Available from: https://pubmed.ncbi.nlm.nih.gov/20666672/ DOI: https://doi.org/10.3109/09546631003686069
Guenova E, Teske A, Fehrenbacher B, et al. Interleukin 23 expression in pyoderma gangrenosum and targeted therapy with ustekinumab. Arch Dermatol [Internet]. 2011 Oct [cited 2022 Mar 9];147(10):1203–5. Available from: https://pubmed.ncbi.nlm.nih.gov/21680759/ DOI: https://doi.org/10.1001/archdermatol.2011.168
Phillips FM, Verstockt B, Sebastian S, et al. Inflammatory cutaneous lesions in inflammatory bowel disease treated with vedolizumab or ustekinumab: an ECCO CONFER multicentre case series. J Crohns Colitis [Internet]. 2020 Oct 1 [cited 2022 Aug 3];14(10):1488–93. Available from: https://pubmed.ncbi.nlm.nih.gov/32318735/ DOI: https://doi.org/10.1093/ecco-jcc/jjaa078
Guillo L, D’Amico F, Danese S, Peyrin-Biroulet L. Ustekinumab for extra-intestinal manifestations of inflammatory bowel disease: a systematic literature review. J Crohns Colitis [Internet]. 2021 Jul 1 [cited 2022 Aug 3];15(7):1236–43. Available from: https://pubmed.ncbi.nlm.nih.gov/33367674/ DOI: https://doi.org/10.1093/ecco-jcc/jjaa260
Feuerstein JD, Ho EY, Shmidt E, et al. AGA Clinical practice guidelines on the medical management of moderate to severe luminal and perianal fistulizing Crohn’s disease. Gastroenterology [Internet]. 2021 Jun 1 [cited 2022 Mar 9];160(7):2496–508. Available from: https://pubmed.ncbi.nlm.nih.gov/34051983/ DOI: https://doi.org/10.1053/j.gastro.2021.04.028
Sulz MC, Burri E, Michetti P, et al. Treatment algorithms for Crohn’s disease. Digestion [Internet]. 2020 Sep 1 [cited 2022 May 6];101 Suppl 1(Suppl1):43–57. Available from: https://pubmed.ncbi.nlm.nih.gov/32172251/ DOI: https://doi.org/10.1159/000506364
Hanauer SB, Sandborn WJ, Feagan BG, et al. IM-UNITI: three-year efficacy, safety, and immunogenicity of ustekinumab treatment of Crohn’s disease. J Crohns Colitis [Internet]. 2020 Jan 1 [cited 2022 May 6];14(1):23–32. Available from: https://pubmed.ncbi.nlm.nih.gov/31158271/ DOI: https://doi.org/10.1093/ecco-jcc/jjz110
Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis [Internet]. 2020 Jan 1 [cited 2022 May 6];14(1):4–22. Available from: https://pubmed.ncbi.nlm.nih.gov/31711158/
Copyright (c) 2023 the Author(s)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.





 https://doi.org/10.4081/dr.2023.9630
https://doi.org/10.4081/dr.2023.9630



