Pityriasis rosea and pityriasis rosea-like eruption after anti-SARS-CoV-2 vaccination: a report of five cases and review of the literature
HTML: 72
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
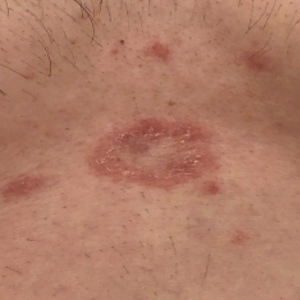
Only a few cases of pityriasis rosea (PR)/pityriasis rosea-like eruption (PRLE) after anti-SARS-CoV-2 vaccination have been reported. In the period May 2021-February 2022 we observed five cases of clinically typical PR that appeared 2 to 3 weeks after anti-SARS-CoV-2 vaccination with BNT162b2 (3 patients) or mRNA-1273 (2 patients). In 4 patients PR appeared after the first vaccination; in one patient after the second one. In 3 patients a biopsy for histopathological examinations was carried out. Results were typical for PR. In all patients laboratory examinations were within normal ranges. All patients were treated with cetirizine. Complete remission was observed within 14-30 days. Four patients were subjected to the second vaccination, but no skin lesions appeared. All patients are currently in good general health. It is possible that a relationship between anti-Sars-CoV-2 vaccination and PR/PRLE exists; however, it is very rare, in consideration of millions of vaccinated subjects and the low number of reported cases of PR/PRLE. The pathogenesis of this relationship is unknown. However, some hypotheses may be advanced: PR/PRLE following anti-Sars-CoV-2 vaccination may be just a coincidence; anti-Sars-CoV-2 vaccines cause a reactivation of HHV-6 and/or HHV-7; vaccines can induce a delayed hypersensitivity response clinically similar to drug-induced PRLE.
McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol 2021;85:46-55.
Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-COV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol 2022;186:142-52.
Carballido Vázquez AM, Morgado B. Pityriasis rosea-like eruption after Pfizer-BioNTech COVID-19 vaccination. Br J Dermatol 2021;185:e34.
Busto-Leis JM, Servera-Negre G, Mayor-Ibarguren A, et al. Pityriasis rosea, COVID-19 and vaccination: new keys to understand an old acquaintance. J Eur Acad Dermatol Venereol 2021;35:e489-91.
Akdaş E, İlter N, Öğüt B, Erdem Ö. Pityriasis rosea following CoronaVac COVID-19 vaccination: a case report. J Eur Acad Dermatol Venereol 2021;35:e491-3.
Cohen OG, Clark AK, Milbar H, Tarlow M. Pityriasis rosea after administration of Pfizer-BioNTech COVID-19 vaccine. Hum Vaccin Immunother 2021;1-2.
McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol 2021;S0190-9622.
Cyrenne BM, Al-Mohammedi F, DeKoven JG, Alhusayen R. Pityriasis rosea-like eruptions following vaccination with BNT162b2 mRNA COVID-19 vaccine. J Eur Acad Dermatol Venereol 2021;35:e546-8.
Farinazzo E, Ponis G, Zelin E, et al. Cutaneous adverse reactions after m-RNA COVID-19 vaccine: early reports from Northeast Italy. J Eur Acad Dermatol Venereol 2021;35:e548-51.
Abdullah L, Hasbani D, Kurban M, Abbas O. Pityriasis rosea after mRNA COVID-19 vaccination. Int J Dermatol 2021;60:1150-1.
Huang L, Yao Z, Zhang J. Two cases of pityriasis rosea after the injection of coronavirus disease 2019 vaccine. J Eur Acad Dermatol 2022;36:e9-e11.
Mehta H, Handa S, Malhotra P, et al. Erythema nodosum, zoster duplex and pityriasis rosea as possible cutaneous adverse effects of Oxford-AstraZeneca COVID-19 vaccine: report of three cases from India. J Eur Acad Dermatol 2022;36:e16-8.
Bostan E, Jarbou A. Atypical pityriasis rosea associated with mRNA COVID-19 vaccine. J Med Virol 2022;94:814-6.
Adya KA, Inamadar AC, Albadri W. Post Covid-19 vaccination papulovesicular pityriasis rosea-like eruption in a young male. Dermatol Ther 2021;34:e15040.
Pedrazini MC, Da Silva MH. Pityriasis rosea-like cutaneous eruption as a possible dermatological manifestation after Oxford-AstraZeneca vaccine: case report and brief literature review. Dermatol Ther 2021;34:e15129.
Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like eruption following COVID-19 vaccination. Front Med 2021;8:752443.
Temiz SA, Abdelmaksoud A, Dursun R, et al. Pityriasis rosea following SARS-CoV-2 vaccination: a case series. J Cosmet Dermatol 2021;20:3080-4.
Marcantonio-Santa Cruz OY, Vidal-Navarro Y, Pesqué D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol 2021;35:e721-2.
Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep 2018;4:800-1.
Copyright (c) 2022 the Author(s)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.





 https://doi.org/10.4081/dr.2022.9503
https://doi.org/10.4081/dr.2022.9503



