Skin reaction to COVID-19 vaccine: a report of 4 cases
Accepted: 12 October 2021
HTML: 10
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
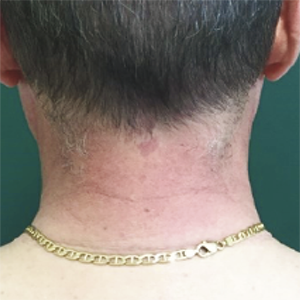
Since the beginning of the covid-vaccine campaign, a lot of local and systemic dermatologic reactions happening after the administration of Coronavirus disease 2019 (COVID-19) vaccines have been described, even if their exact biological mechanism is still debated. In this paper, we report four cases of cutaneous manifestations that arose within ten days after the first dose of messenger RNA (mRNA)-based COVID-19 vaccination: one case of giant urticaria, one case of head and neck redness, and two cases of Erythema multiforme. In our experience, these reactions were mild and transient and all of them resolved, not recurring after the second dose, so these manifestations shouldn’t be considered as an absolute contraindication to the second dose of vaccine, that to date is fundamental.
https://www.aifa.gov.it/web/guest/vaccini-covid-19
Fernandez-Nieto D, Hammerle J, Fernandez-Escribano M, et al. Skin manifestations of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers. 'COVID-arm': a clinical and histological characterization. J Eur Acad Dermatol Venereol 2021;35:e425-7.
McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol 2021;7:S0190-9622.
Farinazzo E, Ponis G, Zelin E, et al. Cutaneous adverse reactions after m-RNA COVID-19 vaccine: early reports from Northeast Italy. J Eur Acad Dermatol Venereol 2021;22:10.
Interim Clinical Considerations for Use of mRNA COVID-19 Vaccines Currently Authorized in the United States. Centers for Disease Control and Prevention. Accessed February 15, 2021. Available at: https://www.cdc.gov/vaccines/covid-19/info-byproduct/clinical-considerations.html)
Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, Harr T. Current perspectives on erythema multiforme. Clin Rev Allergy Immunol 2018;54:177-84.
Zoghaib S, Kechichian E, Souaid K, et al. Triggers, clinical manifestations, and management of pediatric erythema multiforme: A systematic review. J Am Acad Dermatol 2019;81:813-22.
Ayatollahi A, Hosseini H, Firooz R, Firooz A. COVID-19 vaccines: What dermatologists should know? Dermatol Ther 2021;7:e15056.
Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin Immunol 2021;24:108665.
Akinosoglou K, Tzivaki I, Marangos M. Covid-19 vaccine and autoimmunity: Awakening the sleeping dragon. Clin Immunol 2021;3:108721.
Corbeddu M, Diociaiuti A, Vinci MR, et al.Transient cutaneous manifestations after administration of Pfizer‐BioNTech COVID‐19 Vaccine: an Italian single‐centre case series. J Eur Acad Dermatol Venereol 2021;35:e483-e485
Copyright (c) 2022 the Author(s)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.





 https://doi.org/10.4081/dr.2022.9376
https://doi.org/10.4081/dr.2022.9376



