Drug-induced lichenoid exanthema by a vaccine against COVID-19 (Vaxzevria)
Accepted: 12 September 2021
HTML: 550
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
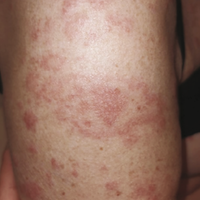
A 66-year-old white female presented with a generalized, erythematous, and itchy eruption for 3 days after. She reported having fever on the first day of eruption, complaints of asthenia, and anorexia with no other systemic symptoms. She received her first dose of Vaxzevria (AstraZeneca, Cambridge, UK) against COVID-19 three weeks prior. The eruption started on the right arm at the vaccine injection site and then spread progressively throughout the entire body. We noticed multiform erythema- like patches with three or four concentric circles with different shades of redness. Anatomopathological analysis indicated a lichenoid histological pattern compatible with adverse event of vaccine. Degressive general corticotherapy was prescribed with an improvement of the symptomatology and complete healing in ten days. Physicians should be aware if this rare adverse event. Drug-induced lichenoid exanthema is considered a non-severe reaction and does not contraindicate the readministration of essential drugs. In this case, the patient refused the second injection of Vaxzevria.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.





 https://doi.org/10.4081/dr.2021.9358
https://doi.org/10.4081/dr.2021.9358



