New therapeutic applications of ozenoxacin in superficial skin infections
Supplementary Files: 364
HTML: 4
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
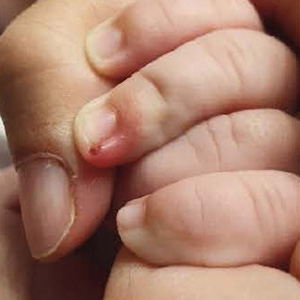
In recent years, the incidence of community-acquired methicillin-resistant S. aureus skin infections (CA-MARSA) has increased in pediatric population without associated risk factors. Ozenoxacin 10mg/g is a topical quinolone that has shown high activity on strains of S. aureus, S. pyogenes and other Gram-positive bacteria sensitive and resistant to methicillin, other quinolones, mupirocin and fusidic acid. Ozenoxacin 10mg/g cream was applied twice a day for 5 days in pediatric patients with superficial skin infections other than non-bullous impetigo where oral antibiotics were not needed. Therapeutic success was achieved in 93.7% of the patients after 5 days of treatment, with a 98.2% decrease in the mean SIRS scale of symptoms. No adverse reaction was reported during treatment. Given the achieved effectiveness, safety, and adherence of the treatment, we believe that pediatricians should consider this topical antibiotic for the treatment of other superficial skin infections, without limiting its use to non-bullous impetigo.
Cobo Vázquez E, Saavedra Lozano J. Infecciones de la piel y partes blandas (I): impétigo, celulitis, absceso (v.3/2019). Guía_ABE. Infecciones en Pediatría. Guía rápida para la selección del tratamiento antimicrobiano empírico. http://www.guia-abe.es. Updated April 7, 2019. Accessed February 23,2021
Larru B, Gerber JS. Cutaneous bacterial infections caused by Staphylococcus aureus and Streptococcus pyogenes in infants and children. Pediatr Clin North Am 2014;61:457-78.
Conejo-Fernández AJ, Martínez-Chamorro MJ, Couceiro JA, et al. Documento de consenso SEIP-AEPAPSEPEAP sobre la etiología, el diagnóstico y el tratamiento de las infecciones cutáneas bacterianas de manejo. Ann Pediatr 2016;84:121.
Williamson DA, Monecke S, Heffernan H, et al. High usage of topical fusidic acid and rapid clonal expansion of fusidic acid-resistant Staphylococcus aureus: a cautionary tale. Clin Infect Dis 2014;59:1451-4.
Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014;59:e10-52.
Fortuny C, Corretger JM, Noguera A, et al. Guía de terapéutica antimicrobiana en pediatría. 3rd ed. Madrid, Spain: Ed. Escofet-Zamora; 2019.
Ozanex (ozenoxacino) 10mg/g crema. Ferrer. Available from: https://cima. aemps.es/cima/dochtml/ft/82357/FT_82357.html Published 2018. Accessed February 7, 2021
Canton R, Morrissey I, Vila J, et al. Comparative in vitro antibacterial activity of ozenoxacin against Gram-positive clinical isolates. Future Microbiol 2018;13:3-19
Morrissey I, Cantón R, Vila J, et al. Microbiological profile of ozenoxacin. Future Microbiol 2019;14:773-87.
López Y, Tato M, Espinal P, et al. In vitro selection of mutants resistant to ozenoxacin compared with levofloxacin and ciprofloxacin in Gram-positive cocci. J Antimicrob Chemother 2015;70:57-61.
López Y, Tato M, Gargallo-Viola D, et al. Mutant prevention concentration of ozenoxacin for quinolone-susceptible or -resistant Staphylococcus aureus and Staphylococcus epidermidis. PLoS One 2019;14:e0223326.
Rosen T, Albareda N, Rosenberg N, et al. Efficacy and safety of ozenoxacin cream for treatment of adult and pediatric patients with impetigo: a randomized clinical trial. JAMA Dermatol 2018;154:806-13.
Bohaty BR, Choi S, Cai C, Hebert AA. Clinical and bacteriological efficacy of twice daily topical retapamulin ointment 1% in the management of impetigo and other uncomplicated superficial skin infections. Int J Womens Dermatol 2015;1:13-20.
Bactroban (mupirocina) 20mg/g pomada. Stiefel Farma S.A. https://cima.aemps.es/cima/dochtml/ft/58868/FT_58868.html Published 2008. Accessed March 3, 2021.
Fucidine (Ácido Fusídico) 20mg/g crema. Leo Pharma. https://cima.aemps.es/cima/dochtml/ft/82357/FT_82357.html Published 2010. Accessed March 3, 2021.
Antonov NK, Garzon MC, Morel KD, et al. High prevalence of mupirocin resistance in Staphylococcus aureus isolates from a pediatric population. Antimicrob Agents Chemother 2015;59:3350-6.
del Rosal T, Méndez-Echevarría A, García-Vera C, et al. Staphylococcus aureus nasal colonization in Spanish children. The COSACO Nationwide Surveillance Study. Infect Drug Resist 2020;13:464351.
Guidance for Industry. Acute bacterial skin and skin structure infections: developing drugs for treatment. draft guidance. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) August 2010 Clinical/Antimicrobial Revision https://www.fda. gov/files/drugs/published/Acute-Bacterial-Skinand-Skin-Structure-Infections—-Developing-Drugs-for-Treatment.pdf
White DG, Collins PO, Rowsell RB. Topical antibiotics in the treatment of superficial skin infections in general practice—a comparison of mupirocin with sodium fusidate. J Infect 1989;18:221-9.
Morley PA, Munot LD. A comparison of sodium fusidate ointment and mupirocin ointment in superficial skin sepsis. Curr Med Res Opin 1988;11:142-8.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.





 https://doi.org/10.4081/dr.2021.9289
https://doi.org/10.4081/dr.2021.9289



