Inflammatory seborrheic keratosis resolution after hyperbaric oxygen therapy: Case presentation and pathophysiology review
Accepted: 2 October 2020
HTML: 27
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
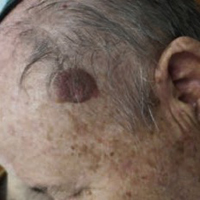
Seborrheic keratosis (SK) is a common epidermal tumor, consisting of a benign proliferation of immature keratinocytes. The natural history of SK is a slow progression over time and complete remission is not expected. The article presents the first case of a complete resolution of a large (2.5 cm diameter) SK lesion after hyperbaric oxygen therapy (HBOT). In addition to the case presentation, the pathophysiology of SK and the potential beneficial physiological effects of HBOT are reviewed and discussed.
Hafner, C. and T. Vogt, Seborrheic keratosis. J Dtsch Dermatol Ges, 2008. 6(8): p. 664-77. DOI: https://doi.org/10.1111/j.1610-0387.2008.06788.x
Schlesinger, T.E. and C. Favre, Enhancing Outcomes in Seborrheic Keratosis: Using a Novel Treatment Solution. J Drugs Dermatol, 2019. 18(7): p. s178-182.
Culbertson, G.R., 532-nm diode laser treatment of seborrheic keratoses with color enhancement. Dermatol Surg, 2008. 34(4): p. 525-8; discussion 528. DOI: https://doi.org/10.1111/j.1524-4725.2007.34098.x
Kim, Y.K., et al., Therapeutic efficacy of long-pulsed 755-nm alexandrite laser for seborrheic keratoses. J Eur Acad Dermatol Venereol, 2014. 28(8): p. 1007-11. DOI: https://doi.org/10.1111/jdv.12231
Polder, K.D., et al., Laser eradication of pigmented lesions: a review. Dermatol Surg, 2011. 37(5): p. 572-95. DOI: https://doi.org/10.1111/j.1524-4725.2011.01971.x
Cheong, K.A. and A.Y. Lee, Guanine Deaminase Stimulates Ultraviolet-induced Keratinocyte Senescence in Seborrhoeic Keratosis via Guanine Metabolites. Acta Derm Venereol, 2020. DOI: https://doi.org/10.2340/00015555-3473
Li, Y., et al., Overexpression of Amyloid Precursor Protein Promotes the Onset of Seborrhoeic Keratosis and is Related to Skin Ageing. Acta Derm Venereol, 2018. 98(6): p. 594-600. DOI: https://doi.org/10.2340/00015555-2911
Wollina, U., Seborrheic Keratoses - The Most Common Benign Skin Tumor of Humans. Clinical presentation and an update on pathogenesis and treatment options. Open Access Maced J Med Sci, 2018. 6(11): p. 2270-2275. DOI: https://doi.org/10.3889/oamjms.2018.460
Tibolla, G., et al., Increased atherosclerosis and vascular inflammation in APP transgenic mice with apolipoprotein E deficiency. Atherosclerosis, 2010. 210(1): p. 78-87. DOI: https://doi.org/10.1016/j.atherosclerosis.2009.10.040
Schulte, E.C., et al., Rare variants in beta-Amyloid precursor protein (APP) and Parkinson's disease. Eur J Hum Genet, 2015. 23(10): p. 1328-33. DOI: https://doi.org/10.1038/ejhg.2014.300
Yoshida, T., et al., The potential role of amyloid beta in the pathogenesis of age-related macular degeneration. J Clin Invest, 2005. 115(10): p. 2793-800. DOI: https://doi.org/10.1172/JCI24635
Cimino, F., et al., Pulsed high oxygen induces a hypoxic-like response in human umbilical endothelial cells and in humans. J Appl Physiol (1985), 2012. 113(11): p. 1684-9. DOI: https://doi.org/10.1152/japplphysiol.00922.2012
Sunkari, V.G., et al., Hyperbaric oxygen therapy activates hypoxia-inducible factor 1 (HIF-1), which contributes to improved wound healing in diabetic mice. Wound Repair Regen, 2015. 23(1): p. 98-103. DOI: https://doi.org/10.1111/wrr.12253
Milovanova, T.N., et al., Hyperbaric oxygen stimulates vasculogenic stem cell growth and differentiation in vivo. J Appl Physiol (1985), 2009. 106(2): p. 711-28. DOI: https://doi.org/10.1152/japplphysiol.91054.2008
Yang, Y., et al., Hyperbaric oxygen promotes neural stem cell proliferation by activating vascular endothelial growth factor/extracellular signal-regulated kinase signaling after traumatic brain injury. Neuroreport, 2017. 28(18): p. 1232-1238. DOI: https://doi.org/10.1097/WNR.0000000000000901
Hadanny, A. and S. Efrati, The Hyperoxic-Hypoxic Paradox. Biomolecules, 2020. 10(6). DOI: https://doi.org/10.3390/biom10060958
Dimitrijevich, S.D., et al., Effect of hyperbaric oxygen on human skin cells in culture and in human dermal and skin equivalents. Wound Repair Regen, 1999. 7(1): p. 53-64. DOI: https://doi.org/10.1046/j.1524-475x.1999.00053.x
Shapira, R., S. Efrati, and U. Ashery, Hyperbaric oxygen therapy as a new treatment approach for Alzheimer's disease. Neural Regen Res, 2018. 13(5): p. 817-818. DOI: https://doi.org/10.4103/1673-5374.232475
Fuller, A.M., et al., Hyperbaric oxygen preconditioning protects skin from UV-A damage. Cell Stress Chaperones, 2013. 18(1): p. 97-107. DOI: https://doi.org/10.1007/s12192-012-0362-2
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.





 https://doi.org/10.4081/dr.2021.8871
https://doi.org/10.4081/dr.2021.8871



