Ustekinumab in the treatment of acute disseminated pyoderma gangrenosum in a patient with Crohn’s disease
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
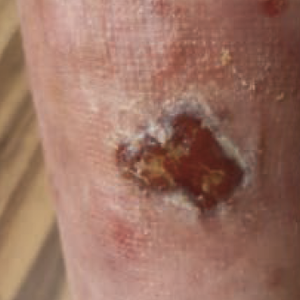
Pyoderma gangrenosum (PG) is an auto-inflammatory dermatosis characterized by lesions that often cause ulcers. We present a case of successful ustekinumab treatment for acute general PG in a 31-year-old woman with coexisting Crohn’s disease (CD). For a month, the patient suffered from skin ulcers, two of them deep and necrotic; a histopathological examination revealed PG. Treatment included: methylprednisolone, azathioprine, betamethasone, gentamicin and zincic ointments, antiseptic compresses, and adalimumab therapy. Due to resistance to the implemented treatment, the patient was enrolled in a clinical trial that included the administration of an anti-cytokines drug, ustekinumab. Subsequently, a significant reduction was observed in the severity of symptoms of PG with no relapse. The use of ustekinumab in patients with PG who have an inadequate response to current treatment or cannot receive first-line treatment can be considered. This applies especially to patients with accompanying autoimmune diseases such as CD.





 https://doi.org/10.4081/dr.2023.9630
https://doi.org/10.4081/dr.2023.9630