Inflammatory seborrheic keratosis resolution after hyperbaric oxygen therapy: Case presentation and pathophysiology review
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
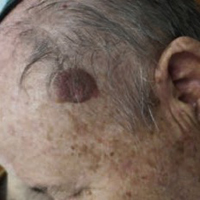
Seborrheic keratosis (SK) is a common epidermal tumor, consisting of a benign proliferation of immature keratinocytes. The natural history of SK is a slow progression over time and complete remission is not expected. The article presents the first case of a complete resolution of a large (2.5 cm diameter) SK lesion after hyperbaric oxygen therapy (HBOT). In addition to the case presentation, the pathophysiology of SK and the potential beneficial physiological effects of HBOT are reviewed and discussed.





 https://doi.org/10.4081/dr.2021.8871
https://doi.org/10.4081/dr.2021.8871