Primary cutaneous adenoid cystic carcinoma of the scalp: dermatosurgical approach with favourable outcome
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
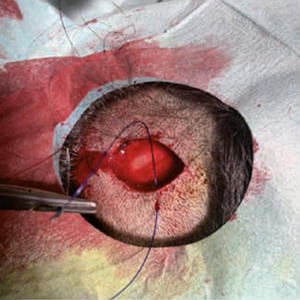
Although described as early as 1975 as a distinct, rare form of cancer with diverse localization, primary cutaneous adenoid cystic carcinoma (PCACC) remains a mystery and challenge for both clinicians and pathologists. The clinical presentation cannot be clearly distinguished from amelanotic melanoma or intradermal nevus, Merkel cell carcinoma, trichofolliculoma, trichoepithelioma or other rare tumors of the adnexa, or dermatofibrosarcoma protuber-ans. The histopathological diagnosis requires not only careful evaluation of standard hematoxylin/eosin preparations, but also immunohistochemical staining with a number of markers such as epithelial membrane antigen (EMA), S-100, SOX-10, Ki-67, CD-117 (c-kit), Vimentin, carcinoembryonic antigen (CEA), Ber-EP4 and many others. The surgical approach should consist of excision with margins between 1 and 2 cm, with the choice of margins depending upon the histopathological findings in the primary excisional specimen. We present a 31-year-old patient with an enlarging, ame-lanotic, plaque-like tumor of the scalp with a duration of no more than 18-24 months. Surgical treatment was performed within two surgical sessions with a total resection field of 1.3 cm. A good cosmetic result was achieved.





 https://doi.org/10.4081/dr.2022.9505
https://doi.org/10.4081/dr.2022.9505