Evans’ syndrome following vaccination with ChAdOx1 nCoV-19 in a patient with new-onset localized scleroderma
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
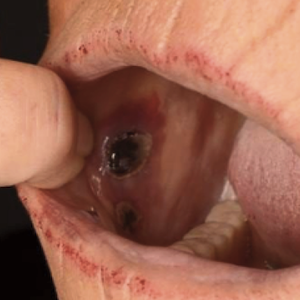
Growing evidence suggests that COVID-19 vaccines can induce hematological conditions. Here, we report a case of Evans’ syndrome, a combination of immune thrombocytopenic purpura and autoimmune hemolytic anemia following administration of the ChAdOx1 nCoV-19 vaccine. The present case further supports the notion that COVID-19 vaccines can trigger in rare cases severe persistent autoimmune-mediated hematological conditions which may predominantly occur in patients with underlying autoimmune conditions.





 https://doi.org/10.4081/dr.2022.9470
https://doi.org/10.4081/dr.2022.9470