Long term efficacy, safety, and tolerability of tildrakizumab in epileptic patient with psoriasis and eczema
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
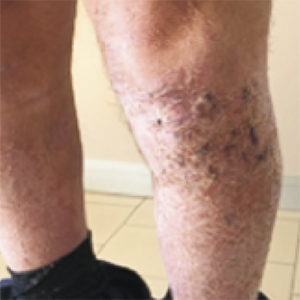
Psoriasis is a chronic inflammatory disease which mostly affects skin. Tildrakizumab is a specific anti-interleukin-23p19 monoclonal antibody approved for the treatment of plaque psoriasis in adults. Herein, we report about a patient who came to our attention for a moderate-to-severe plaque psoriasis, involving primarily upper limbs, elbow, abdomen and knees (PASI 18 – DLQI 22). His medical history was relevant for epilepsy controlled pharmacologically. In addition, an eczematous and edematous appearance of the tibial area was detected; the histologic findings did not contradict the diagnostic hypothesis of subacute spongiotic dermatitis. The patient was treated with Tildrakizumab. After 12 weeks the clinical lesions improved significantly, and the eczematous component disappeared in the tibial area bilaterally. The clinical improvement was maintained even after one year of therapy. Tildrakizumab showed excellent results in the control of psoriasis, with an excellent safety profile. The promising results of clinical trials have been confirmed in a real-life setting. There are no reports about its safety in epileptic patients. In our case, neurological adverse events did not verify and tildrakizumab managed to control both the psoriatic and eczematous components





 https://doi.org/10.4081/dr.2022.9447
https://doi.org/10.4081/dr.2022.9447