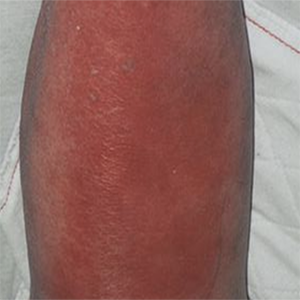
The effectiveness of erysipelas prophylaxis depends on the cumulative dose of benzathine penicillin G
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Erysipelas is an acute infection due to S. pyogenes and is characterized by a high risk of relapses. The number of patients suffering from one or more recurrences varied depending on the study and accounted for between 16% and 47% of the total number of those affected. Antibiotic prophylaxis with the use of penicillin can reduce the risk of recurrence by 47%. A number of 873 patients with erysipelas treated at the Hospital for Infectious Diseases in Warsaw from 2010 to 2018 was enrolled in the study. Benzathine-penicillin G was given intramuscularly at a dose of 1.2 MU or 2.4 MU or 3.6 MU. The earliest moment that prophylactic treatment was administered was the first episode of erysipelas recurrence. The decision to administer the antibiotic and the dose to use was discretionally made by the examining physician. Altogether 104 (11.9%) persons experienced at least one episode of erysipelas recurrence during the study period. A total of 2976 doses of benzathine- penicillin G (BP) were administered. The most common dose was that of 2.4 MU (2380, 80%). The dose of 1.2 MU was given 567 times (19%). The highest dose, i.e. 3.6 MU, was administered to only 5 patients (8 applications, 0.2%). No effect was shown by either the number of benzathine- penicillin G administered doses (p=0.07) or the median dose (p=0.65), whereas patients without relapse received a statistically higher cumulative dose of the antibiotic (p=0.047). Age was a risk factor of recurrence only in the group of diabetic patients (p=0.03). Benzathine penicillin G given in an appropriate cumulative dose is effective in preventing erysipelas recurrence.





 https://doi.org/10.4081/dr.2022.9429
https://doi.org/10.4081/dr.2022.9429