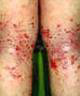
Photocarcinogenicity of selected topically applied dermatological drugs: calcineurin inhibitors, corticosteroids, and vitamin D analogs
12
0
10
0
 Smart Citations
Smart Citations12
0
10
0
Citing PublicationsSupportingMentioningContrasting
See how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.





 https://doi.org/10.4081/dr.2010.e13
https://doi.org/10.4081/dr.2010.e13